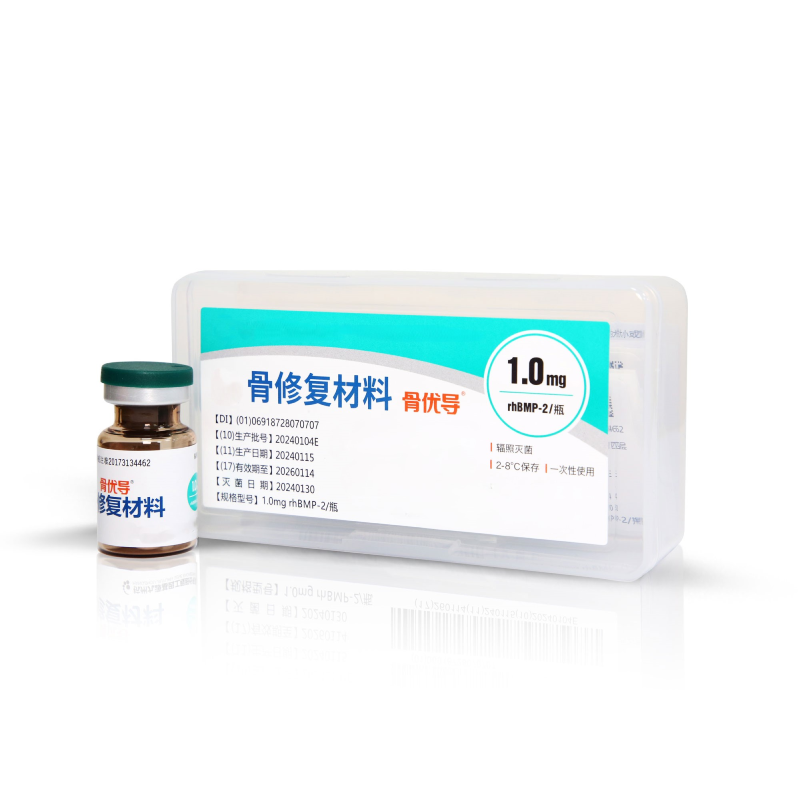
Bone Repair Material
|
Product Name |
Bone Repair Material |
|
Model Specification |
0.3mg rhBMP-2/vial, 0.5mg-I rhBMP-2/vial, 0.5mg-II rhBMP-2/vial, 0.8mg rhBMP-2/vial, 1.0mg rhBMP-2/vial, 1.2mg rhBMP-2/vial, 1.5mg rhBMP-2/vial, 2.0mg, rhBMP-2/vial, 2.4mg rhBMP-2/vial, 3.0mg rhBMP-2/vial, 1.6mg rhBMP-2/vial, 0.4mg, rhBMP-2/vial, 0.4mg-I rhBMP-2/vial, 0.6mg rhBMP-2/vial, 0.6mg-I rhBMP-2/vial, 0.75mg, rhBMP-2/vial, 0.8mg-I rhBMP-2/vial, 1.0mg-I rhBMP-2/vial |
|
Structural Component |
The product is using recombinant human bone morphogenetic protein-2 (rhBMP-2) as the active ingredient and using medical gelatin, soy lecithin and hydroxyapatite as the carrier materials. It is of sterile packaging |
|
Product Property |
The product can induce directional differentiation of intracorporal mesenchymal stem cells into osteoblasts, after filled at the defect area of bone tissue, osteogenesis can be induced to stimulate the formation of bone and cartilage, so as to achieve tissue repair. It could be degraded and absorbed by the body |