Intraosseous Infusion System Instructions for Use
Ⅰ. DEVICE NAME
The device name is Intraosseous Infusion System.
Ⅱ DESCRIPTION
The system include the puncture needle and the Orthopedic clamp holder.
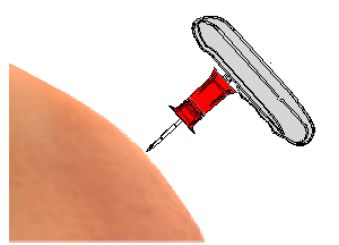
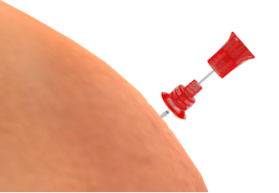
Figure 1. The puncture needle position
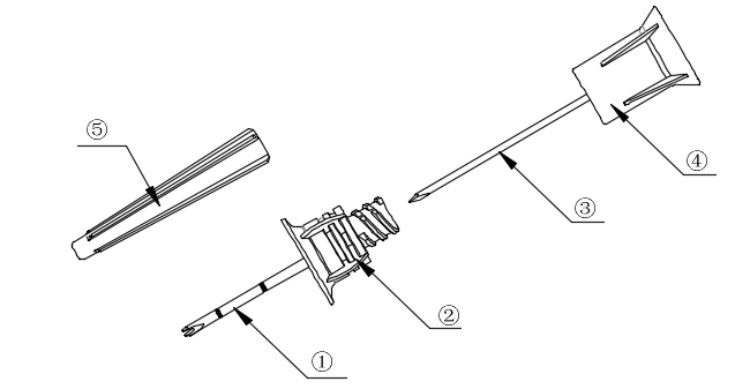
①-Needle tubing ②-Needle tubing base ③-Needle core ④-Needle core base
⑤-Needle cover
Figure 2. The orthopedic clamp holder
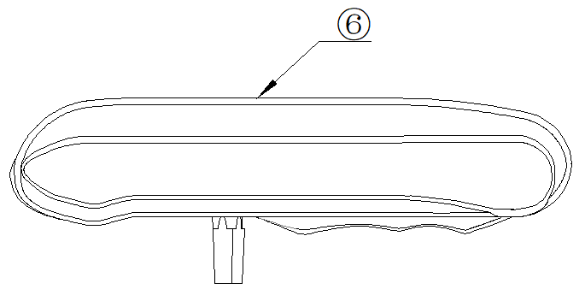
⑥-Orthopedic clamp holder
III AVAILABLE PRODUCT SIZES AND PRODUCT SPECIFICATION
|
No. |
Catalog Numbers |
Specification |
Nominal Needle Length(mm) |
Puncture Needle Outside Diameter |
|
1 |
MN100 |
45mm×15G |
45 |
15G(1.8mm) |
|
2 |
MN101 |
25mm×15G |
25 |
|
|
3 |
MN102 |
15mm×15G |
15 |
Ⅳ DESCRIPTION
|
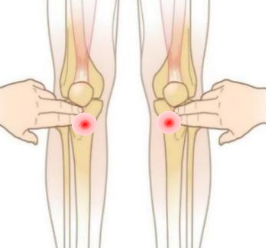
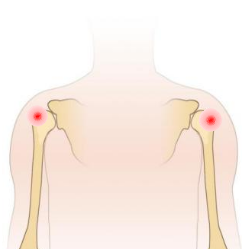
1.Determine the puncture site (preferably proximal tibia) and disinfect the puncture site
|
||||
|
2. Select proper size puncture needle according to the operation requirements (15 / 25 / 45mm×15G)..Remove the needle cover.And connect the orthopedic gripper with the puncture needle.
|
|
|||
|
3.Make the puncture needle penetrates the subcutaneous tissue until contacting the bone (common puncture position: proximal tibia or proximal humerus),then press and rotate the Orthopedic clamp holder with slight force. |
|
|||
|
4.When the puncture needle reaches the bone marrow cavity and then remove the orthopedic gripper.
|
|
|||
|
5.Remove the needle core . Connecting the syringe with the needle tubing base . If the 20ml syringe which containing 10ml normal saline can aspirate the bone marrow fluid, it indicates that the infusion can be carried out. Then rinsing with the normal saline in the syringe. |
|
|||
|
6.Connecting any standard 6% luer tap set for infusion. Using the 300mmHg pressurized infusion can improve the infusion speed. |
|
|||
Ⅴ INDICATIONS FOR USE
Used to establish channel to the tibia or humerus.
Ⅵ CONTRAINDICATIONS
- Patients with fracture.
- Too much tissue or lack of enough anatomical markers.
- Infection of insertion site.
- The insertion site has undergone major orthopaedic surgery.
- The insertion site has undergone intraosseous puncture, prosthetic limb or prosthetic joint within the past 24 hours.
Ⅶ WARNINGS
- The use of the intraosseous infusion system is restricted to skilled medics, nurses, paramedics and doctor who were trained on the device.
- Please use it within the validity period. Please read the operation manual carefully before use. It is forbidden to use,if the package is damaged.
- The safe use of the intraosseous infusion system in patients with osteoporosis, osteopetrosis, Osgood-Schlatter disease, or other tibial bone pathology or deformity has not been proven. These conditions may obscure landmarks of the tibia/ humerus
- The syringe and puncture needle must be dry to avoid hemolysis.
- The needle head of the puncture needle shall not swing too much after entering the bone to avoid breaking.
- Please destroy the puncture needle after use.
- Metal needle are not MRI compatible.
- Do not aim the puncture needle toward the joint space or epiphysial plate.
Ⅷ STERILIZATION VALIDITY & LIFESPAN
The puncture needle:36 months & lifespan see outer package. Used by date.
Ⅸ SYMBOL DESCRIPTION
|
|
Manufacturing date |
|
Used by date |
|
|
Lot number |
|
Product Specification |
|
|
Sterilized with ethylene oxide gas |
|
Do not use if this package is open or damaged |
|
|
For one use only |
|
Caution: Attention see Instructions for Use |
|
|
Warning |
|
Not Raining |
Ⅹ NAME AND ADDRESS OF AFTER-SALES SERVICE AND MANUFACTURING COMPANY
Name of after-sales service/manufacturing company: Suzhou Amathes Medical Technologr CO.,Ltd
After-sales service/manufacturing address: 218 Xinghu St., #A6-102, Suzhou, Jiangsu, P.R.China
Tel:0512-65975508
Zip code:215000