Semaglutide
|
Product Name |
Semaglutide |
|
Cas No. |
910463-68-2 |
|
Sequence |
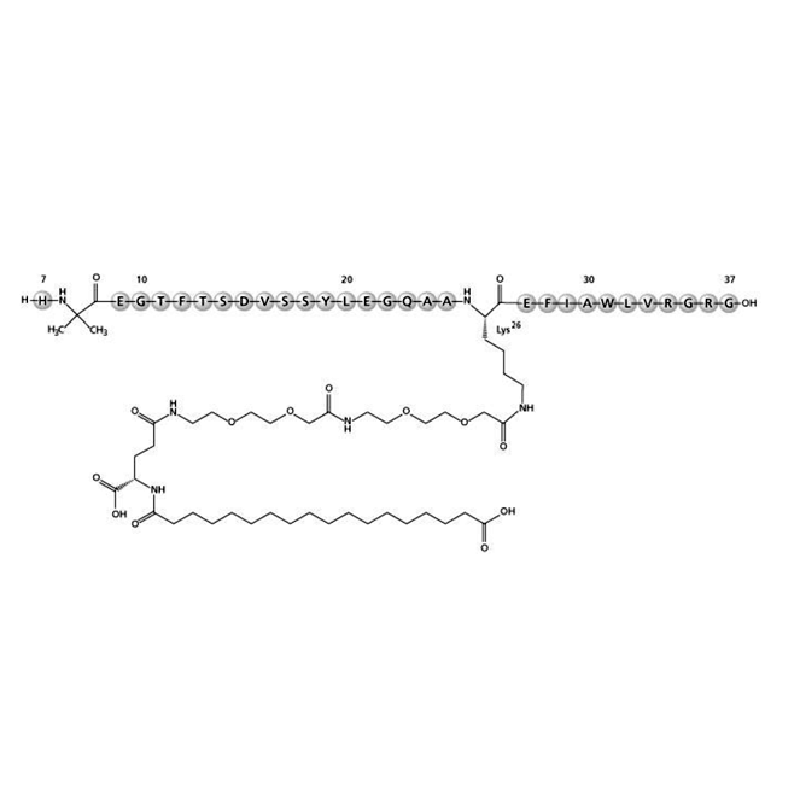
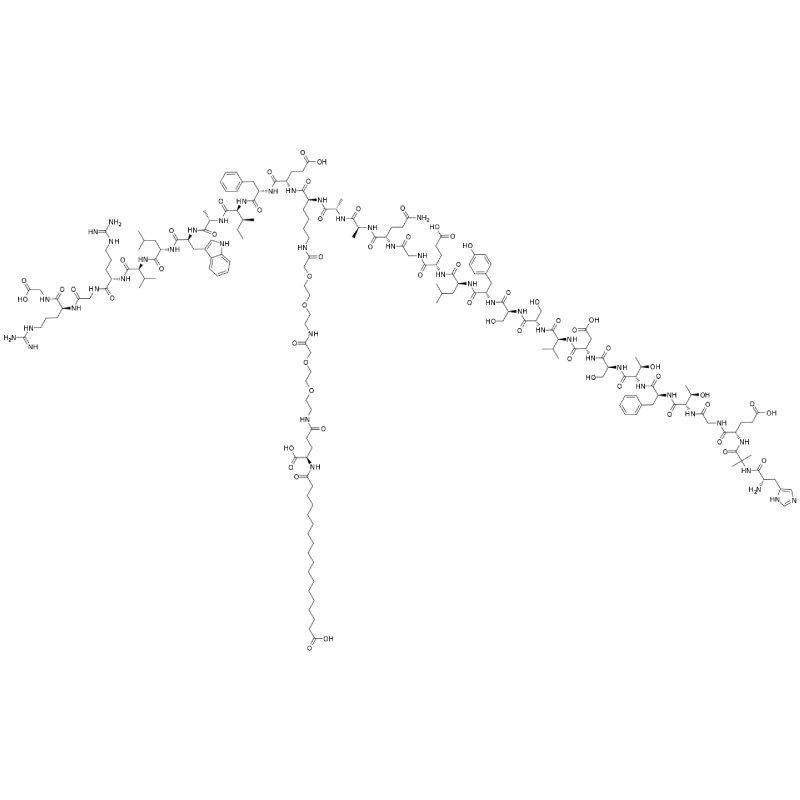
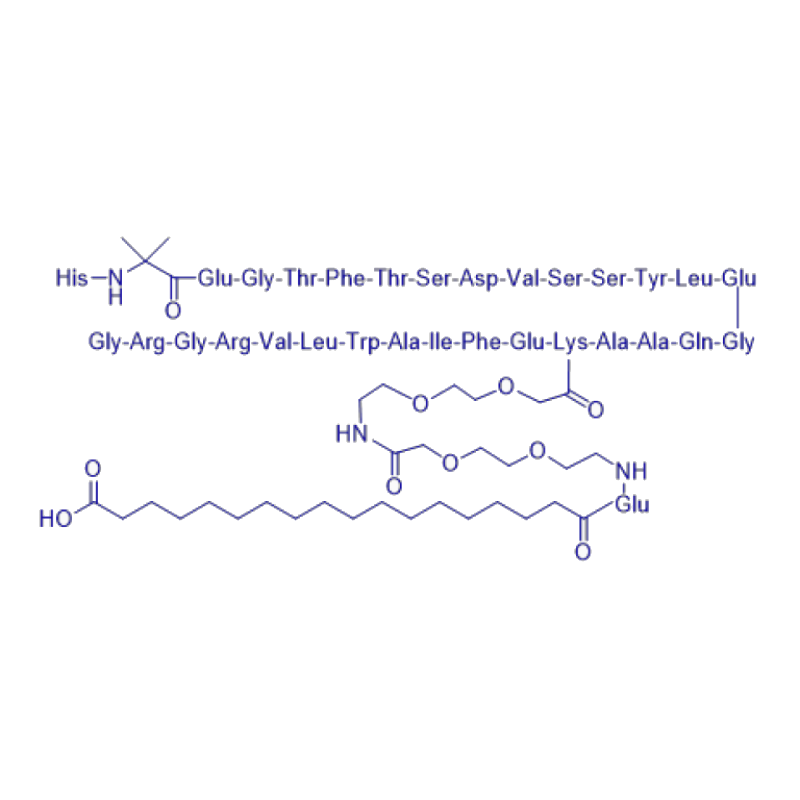
His-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys(PEG2-PEG2-γ-Glu-17-carboxyheptadecanoyl)-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly |
|
Molecular Formula |
C187H291N45O59 |
|
Molar Mass |
4,113.58 Da |
|
Purity |
/ |
|
The Maximum Unspecified Impurity |
≤0.1% |
|
Storage Temperature |
2-8℃ |
|
Packing Size |
50G/Bottle or at customers requirement. |
Label: semaglutide peptide GLP-1
Semaglutide is a long-acting GLP-1 receptor agonist launched by Denmark's Novo Nordisk after Liraglutide. It is the second GLP-1 receptor agonist in the world to obtain dual indications for obesity and type II diabetes. Compared with Liraglutide , Semaglutide has a longer half-life of 165 hours (nearly 7 days) and can be injected once a week. Semaglutide was first launched in the United States on December 5th, 2017, and was launched in China in April 2021.
Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in patients with type II diabetes; It is indicated to reduce major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction or nonfatal stroke) risk. In addition, on June 4th, 2021, Semaglutide injection (2.4 mg, once weekly) was officially approved by the US FDA for chronic weight management in obese or overweight adults. Recently, FDA approved the expanded indication of Semaglutide for the treatment of obesity in adolescents aged 12 years and above.
October 10th, 2023, Novo Nordisk announced the Semaglutide interim analysis results of the FLOW trial in preventing renal dysfunction who are diabetes patients. Results showed that Semaglutide was significantly effective in delaying the progression of chronic kidney disease, leading the independent Data Monitoring Committee (DMC) to recommend early termination of the trial based on specific predetermined criteria.
According to the performance announced by Novo Nordisk , Semaglutide has the most outstanding sales data, with total sales in 2022 reaching US$10.9 billion, and revenue in China of RMB2.085 billion approximately. Sales in the first three quarters of 2023 have exceeded US$14.7 billion, of which the weight loss drug ( Wegovy ) has a year-on-year growth rate of 481%, the diabetes injection form ( Ozempic ) has a year-on-year growth of 53%, and the diabetes oral dosage form ( Rybelsus ) has a year-on-year growth of 77%. All are still growing at a rapid pace, with full-year sales expected to exceed US$20 billion.