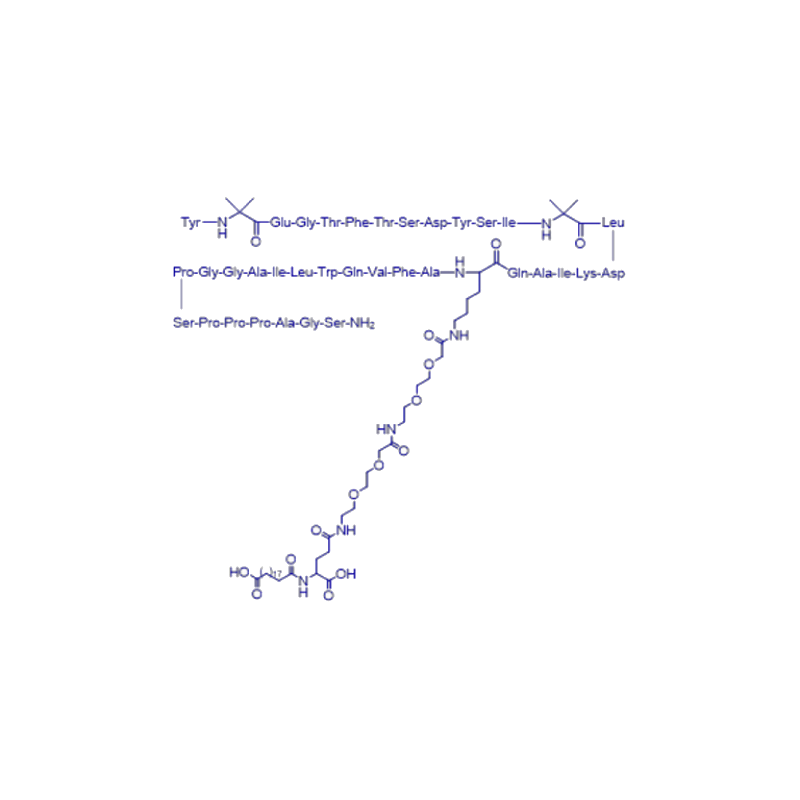
Tirzepatide
Tirzepatide is a blockbuster GLP-1 and GIP dual-target agonist developed by Lilly after Dulaglutide. It is a modified peptide containing 39 amino acids, with a C20 fatty acid group, and can interact with Albumin binds and extends half-life to 5 days. It was launched in the United States in May 2022, with sales reaching US$480 million by the end of 2022.
Currently, Tirzepatide has been approved for use in type II diabetes in the United States, Europe, and Japan, meanwhile launch requests have also been accepted in China. In October 2022, the FDA granted Tirzepatide as a Fast Track designation for adults with obesity or overweight, and it is expected to complete the rolling submission of marketing applications for obesity/overweight indications in the United States soon.
According to Lilly's first quarter report of 2023, Tirzepatide's sales is US$568 million. Although it was inferior to Dulaglutide and Semaglutide in sales amount,but its requirement quantity and sales volume will be exceeded the latter two items after launching. As of now, its sales revenue in the first three quarters has reached US$2.96 billion.